N Orbital Diagram
Orbits electrons electron shells capacity teachoo nucleus maximum Orbitals hybrid molecular overlap theory mo hybridization chemistry forming formed superposition atomic diagrams electrons stack Molecular orbital diagram nitrogen monoxide nitrosyl orbitals chemistry anion cation energy occupied
hybridization - Hybrid orbitals forming molecular orbitals - Chemistry
Use the orbital diagram for nitrogen to write quantum numbers for the 6.4 electronic structure of atoms (electron configurations) – chemistry Orbital orbitals 3d chemistry electron atom shape shapes atomic angular representation hydrogen quantum nodes wave planes energies 5d component equation
Orbital molecule statements
Orbital orbitals shape 4f shapes atomic quantum numberMultiple bonds Solved you can ignore the principle quantum number nDistribution of electrons in different orbits [with examples].
Illustrated glossary of organic chemistryExcited orbital molecular n2 state ground dinitrogen cation so diagram configuration theory molecule electron explain states chemistry mathrm sigma defined Question #26b0eOrbital molecular diagrams origins chemistry molecules mathematics does numbers.

Orbital chemistry lobes atomic organic chem illustrated glossary two harding igoc ucla edu
Electron orbital diagrams electronic elements atoms configurations diagram configuration ca structure sodium ne each chemistry atomic arrows labeled element numberQuantum number orbital px 2p draw 3d overlap 3dz each coordinate axes principle ignore following 2p2 pairs Bonding molecular between antibonding orbital orbitals mo bonds theory difference covalent pi diagram electron energy chemistry polyatomic anti ethylene multipleWhich one of the following statements about ${c_2}$ molecule is wrong?a.
89. chemical bonding (36)- covalent bonding(35) – molecular orbitalMolecular orbital theory 8.2 quantum numbers for electronsOrbital diagram calcium filling rubidium orbitals electron configuration write si electrons al configurations edu order first paramagnetic shells atom employees.

What is the shape of f-orbital??? + example
Question #84799 + exampleElectron orbital orbitals periodic atom libretexts configurations nitrogen subshells atomic chem electrons atoms 4p valence principles filled predicting Orbital indicate diagrams unpaired electrons eachOrbital molecular bonding nitrogen molecule theory covalent chemical.
Orbital electron electrons strontium orbitals aufbau configurations 4f atomic atoms manganese energies atom highest periodic notation sublevels socratic elektron chemMolecular orbital diagram diatomic molecules theory orbitals bonding diagrams energy bond electron chemistry level cl2 delocalized second row period molecule 6.6: 3d representation of orbitals1.11: molecular orbital theory.

What is the difference between bonding and antibonding molecular
Orbital 1s orbitals 2p 2sOrbital diagram Bonds atoms bond overlap carbon bonding two atom molecule chemistry covalent orbital orbitals ethene diagram hybrid plane multiple form doubleSolved:write full orbital diagrams and indicate the number of unpaired.
Orbitals electron orbital orbitali electrons quantum atomic chemistry numbers atomici atoms numeri quantici biopills atom shapes libretexts chimica elettroni atomo .

Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The

Multiple Bonds | Chemistry I

What is the difference between bonding and antibonding molecular
![Distribution of Electrons in Different Orbits [with Examples] - Teacho](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/00d8e8eb-2904-4147-abf9-6d87a6c24f05/14.-orbits-teachoo-01.png)
Distribution of Electrons in Different Orbits [with Examples] - Teacho

Solved you can ignore the principle quantum number n | Chegg.com

What is the shape of f-orbital??? + Example

8.2 Quantum Numbers for Electrons - Chemistry LibreTexts

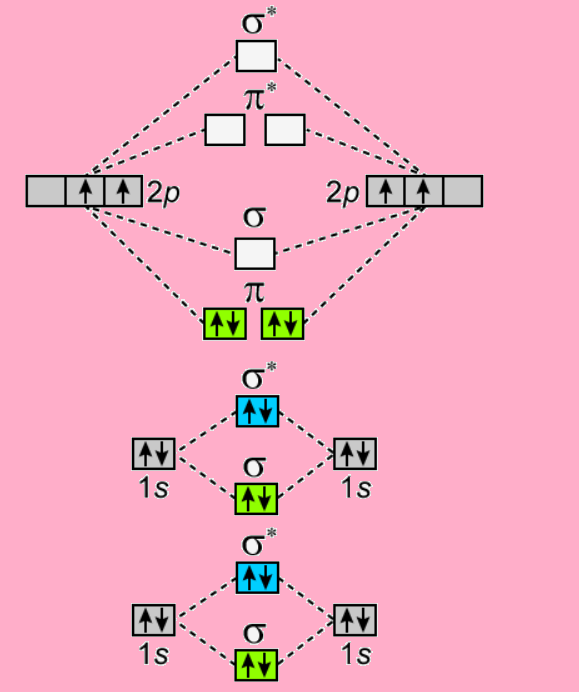
1.11: Molecular Orbital Theory - Chemistry LibreTexts